Hidrólisis enzimática in vitro y microscopia electrónica de la harina horneada y extrudida de arracacha
Resumen
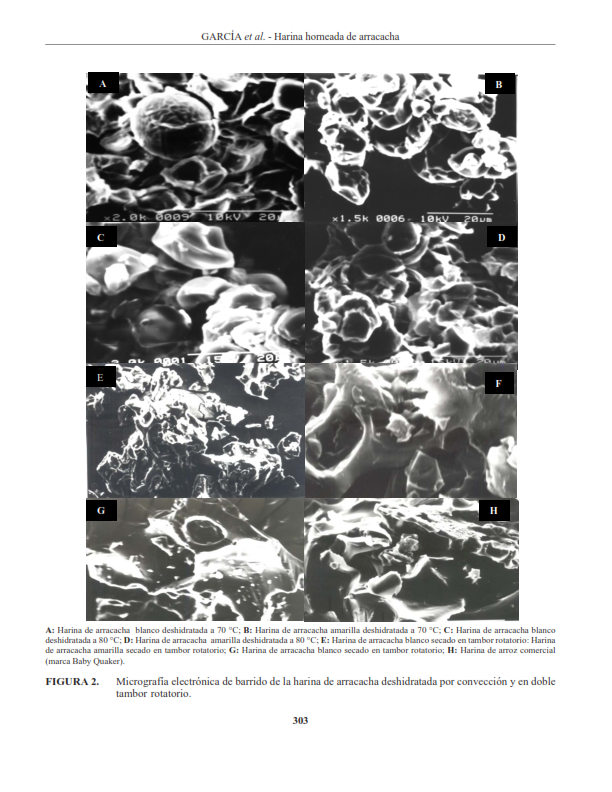
Con la finalidad de analizar el efecto de los tratamientos térmicos de secado sobre la hidrólisis enzimática in vitro del almidón en las harinas de arracacha, Arracacia xanthorrhiza Bancroft, de los morfotipos blanco y amarillo, se obtuvieron las harinas por los métodos de convección a la temperatura de 70 a 80 ºC y en un secador de doble tambor rotatorio a la presión de vapor de 40 Psi. Observando, los cambios en la estructura de los gránulos de almidón a través de micrografías vistas en el microscopio electrónico de barrido (MEB). Los resultados demostraron que las harinas de ambos morfotipos fueron sensibles a la hidrólisis de la enzima α-amilasa pancreática, resultando las obtenidas en doble tambor rotatorio de alta digestión enzimática con respecto a las horneadas por el método de convección, a causa de la mayor incidencia del tratamiento térmico de secado sobre los cambios estructurales a nivel de las regiones amorfas de los gránulos de almidón, que vistos al MEB demostraron la pérdida de birrefringencia del mismo.
Descargas
Citas
• Buléon, A., P. Colonna, V. Planchot and S. Ball. 1998. Starch granules: Structure and biosynthesis. International Journal of Biological Macromolecules, 23:85-112.
• Engliyst, H. and J. Cummings. 1985. Digestion of the polysaccharides of some cereal foods in the human small intestine. American Journal of Clinical Nutrition. 42:778-787.
• Englyst, H., S. Kingman and J.Cummings. 1992. Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition, 46 (suppl. 2):33-50.
• Espín, S., X. Chiriboga y J. Altamirano. 2002. Caracterización química y digestibilidad de los almidones de siete especies de raíces y tubérculos andinos del Ecuador. In: resúmenes XI Congresso Italo-Latinoamericano di Etnomedicina"Alberto Di Capua" Atti_Resumenes.Universitá degli Studi di Pavia. Pavia, Italia. p.155.
• Farhat, J., A. Becker, B. Valles, R. Neale and S. Hill. 2001. Effect of the extent of conversion and retrogradation on the digestibility of potato starch. Starch/Stärke, 53:431-436.
• Gallant, I., A. Bouchet, S. Buleón and S. Pérez.1992. Physical characteristics of starch granules and susceptibility to enzymatic degradation. European Journal Clinical Nutrition, 46(1):3-16.
• Goñi, I., L. García and F. Saura. 1996. Analysis of resistant starch: a method for food products. Food Chemistry,56(4):445-449.
• González, Z. y E. Pérez. 2003. Evaluación fisicoquímica y funcional de almidones de yuca (Manihot esculenta Crantz) pregelatinizados y calentados con microondas. Acta Científica Venezolana, 54:127-137.
• Gunaratne, A. and R. Hoover. 2002. Effect of heat- moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydrate Polymers, 49(4):425-437.
• Gupta, R., G. Paresh, H. Mohapatra, V. Kumar and B. Chauhan. 2003. Microbial α-amylases: a biotechnological perspective. Process Biochemistry, 38(11):1 599-1 616.
• Holm, J., I. Bjorck, A. Drews and N. Asp. 1986. A rapid method for the analysis of starch. Starch/Starke, 38:224-229.
• Hoover, R. 2001. Composition, molecular, structure and physicochemical properties of tuber and root starches. Review. Carbohydrate Polymers, 45:253-267.
• Hoover, R., and Y. Zhou. 2003. In vitro and in vivo hydrolysis of legume starches by α- amylase and resistant starch formation in legumes. A review. Carbohydrate Polymers, 54(4):401-417.
• Hung, P. and N. Morita. 2005. Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydrate Polymers, 61(3):314-321.
• Islas, J., R. Rendón, E. Agama, F. Gutiérrez, J. Tovar, G. Arámbula and L. Bello. 2006. In vitro digestion rate and resistant starch content of tortillas stored at two different temperatures. Food Science and Technology. 39(8):947-951.
• Jayakody, L., R. Hoover, Q. Liu and E. Donner. 2007. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp.) starch grown in Sri Lanka. Carbohydrate Polymers. 69:148-163.
• Karisson, M. and E. Eliasson. 2003. Gelatinization and retrogradation of potato starch in situ as assessed by differential scanning colorimetry. Lebensm Wiss Technology. 36:735-741.
• Laurentín, M., J. Cárdenas, E. Ruales, E. Pérez and J. Tovar. 2003. Preparation of indigestible pyrodextrins from different starch sources. Journal of Agricultural and Food Chemistry, 51:5 510-5 515.
• Leonel, M., A. Dal Bianco and M. Reis. 2004. Physicochemical and microscopical characterizations of sweet potato, canna, cassava and cocoyam starches and their expansion properties after photochemical modification. Brazilian of Journal Food Technology, 7(2):129-137.
• Liu, Q., E. Donner, Y. Yin, R. Huang and M. Fan. 2006. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in China. Food Chemistry, 99(3):470-477.
• Lynn, A. and M. Cochrane. 1997. An evaluation of confocal microscopy for the study of starch granule enzymic digestion. Starch/ Stärke, 49:106-111.
• Montgomery, D. 1991. Diseño y análisis de experimentos. Editorial Iberoamericana. Caracas. 589 p.
• Moorthy, S. 2002. Physicochemical and functional properties of tropical tubers starch. A review. Starch/ Stärke, 54:559-564.
• Osorio, P., E. Agama, R. Carmona, J. Tovar, O. Paredes and L. Bello. 2004. Resistant starch and in vitro starch digestibility of cooked "ayocote" bean (Phaseolus coccineus). Revista Interciencia, 29:510-514.
• Pacheco, E. 2001. Evaluación nutricional de sopas deshidratadas a base de harina de plátano verde digestibilidad in vitro del almidón. Acta Científica Venezolana, 52:278-282.
• Pandey, A., P. Nigam, C. Zoccol, V. Soccol, D. Singht and R. Mohan. 2000. Advances in microbial amylases. Biotechnology and Applied Biochemistry, 31(2):135-152.
• Polaina, J. 2004. Structure, function and molecular engineering of enzymes involved in carbohydrate digestion. Revista Mensaje Bioquímico, México. 28:61-76.
• Planchot, P., D. Colonna, J. Gallant and B. Bouchet. 1994. Extensive degradation of native starch granules by α- amylase from Aspergillus fumigatus. Journal of Cereal Science, 21:163-171.
• Prosky, L., N. Asp, E. Schweiser, J. Devries and Y. Furda. 1992. Determination of insoluble, soluble and total dietary fiber in foods and food product. Interlaboratory study. Journal Association Official Analytical Chemistry, 75:1 017-1 023.
• Rao, M. and J. Tattiyakul. 1999. Granule size and rheological behavior of heated tapioca starch dispersions. Carbohydrate Polymers. 38:123-132.
• Rendleman, J. 2000. Hydrolytic action of a-amylase on high amylose starch of low molecular mass. Biotechnology Applied Biochemistry. 31:171-178.
• Rodríguez, D., M. Espitia, Y. Caicedo, Y. Cordoba, Y. Baena y C. Mora. 2005. Caracterización de algunas propiedades fisicoquímicas y farmotécnicas del almidón de arracacha (Arracacha xanthorriza). Revista Colombiana de Ciencias Químicas y Farmacéuticas, 34(2):140-146.
• Rodríguez, L. and S. Silva. 2003. Resistant starch and its physicochemical properties. Revista de Nutrición, 16(2):219-226.
• Ruiz, G. 2006. Obtención y caracterización de un polímero biodegradable a partir del almidón de yuca. Revista Ingeniería y Ciencia, 2(4):5-28.
• Sandoval, A., E. Rodríguez y A. Fernández. 2005. Aplicación del análisis por calorimetría diferencial de barrido para la caracterización de las modificaciones del almidón. Revista de la Facultad de Minas- Colombia (DYNAS), 72(146):45-53.
• Serrano, P. and C. Franco. 2005. Annealing and enzymatic hydrolysis of cassava starch. Brazilian of Journal Food Technology, 8(3):220-232.
• Shamai, K., H. Bianco and E. Simón. 2003. Polymorphism of resistant starch type III, Carbohydrate Polymers, 54:363-369.
• Sreenath, H. 1992. Studies on starch granules digestion by a-amylase. Starch/Stärke, 44:61-63.
• Svihus, B., A. Uhlen and O. Harstad. 2005. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Animal Feed Science and Technology, 122:303-320.
• Tester, R. and S. Debon. 2000. Annealing of starch. a review. Journal of Biological Macromolecules, 27:1-12.
• Tester, R., X. Qi and J. Karkalas. 2006. Hydrolysis of native starches with amylases. Review. Animal Feed Science and Technology, 130(1-2):39-54.
• Tovar, J., A. de Francisco, I. Bjorck and N. Asp. 1991. Relationship between microstructure and in vitro digestibility of starch in precooked leguminous seed flours. Food Structure, 10:19-26.
• Tovar, J., C. Melito, E. Herrera, A. Rascón and E. Pérez. 2002. Resistant starch formation does not parallel syneresis tendency in different starch gels. Food Chemestry, 76:455-459.
• Tovar, J., I. Bjorck and N. Asp. 1990. Starch content and amylolysis rate in precooked legume flours. Journal of the Agriculture and Food Chemistry, 38:1 818-1 823.
• Tovar, J., M. Fernández and A. Blanco. 2005. Digestibilidad in vitro del almidón en preparaciones cocidas y molidas de frijol (Phaseolus vulgaris L.). Revista Interciencia, 30(12):780-783.
• Van Den Einde, R., A. Van Der Goot and R. Boom. 2003. Understanding molecular weight reduction of starch during heating-shearing processes. Journal of Food Science, 68(8):2 396-2 404.
• Wang, W., J. Powell and A. Oates. 1997. Effect of annealing on the hydrolysis of sago starch granules. Carbohydrate Polymers, 33:195-202.
• Yadav, A., M. Guha, R. Tharanathan and S. Ramteke. 2006a. Changes in characteristics of sweet potato flour prepared by different drying techniques. Food Science and Technology, 39(1):20-26.
• Yadav, A., M. Guha, R. Tharanathan and S. Ramteke.2006b. Influence of drying conditions on functional properties of potato flour. Journal European Food Research and Technology, 223(4):553-560.
• Zhang, T. and C. Oates. 1999. Relationship between α-amylase degradation and physical-chemical properties of sweet potato starches. Food Chemistry, 65:157.