Detection of RAPD polymorphisms in Musa sp. materials with differential response to the attack of Xanthomonas campestris pv. musacearum
Abstract
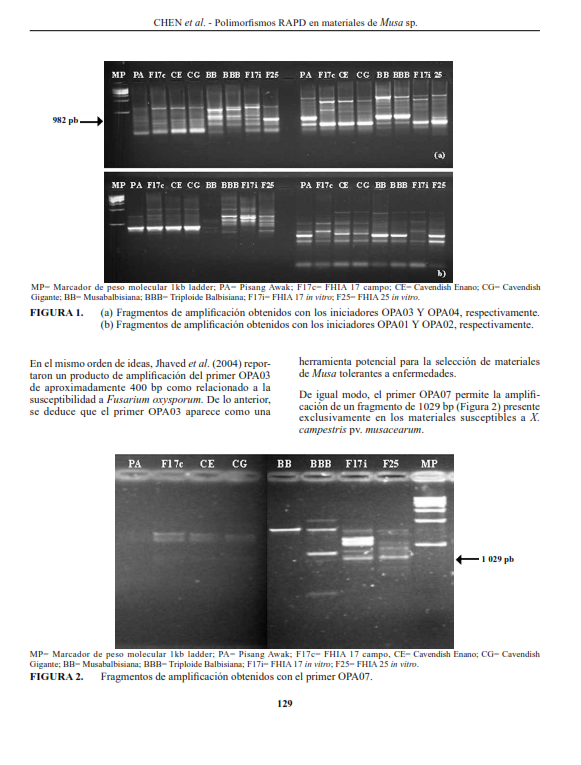
To detect RAPD polymorphisms in Musa sp. with differential response to Xanthomonas campestris pv. Musacearum six susceptible genotypes (Pisang Awak, Dwarf Cavendish, Giant Cavendish, FHIA 17, FHIA17 in vitro, FHIA25) and two tolerant (BB and BBB) were evaluated. Conditions for DNA isolation and random amplification of polymorphic DNA (RAPD) were established. DNA was isolated according to Dellaporta et al. (1983), as amended by CIAT (2002), DNA concentration was measured spectrophotometrically. Additionally, A260/A280 and A260/A230 ratios indicated the purity of the molecule. 27 Operon Technology primers (20 sequences in the series OPA, 5 from OPJ and 2 from OPM) were used. The statistical analysis was performed with a hierarchical cluster analysis using Infostat Professional version 2.0, Ward clustering method and the Jaccard distance. Specific band patterns were obtained for each genotype. Six bands were identified as unique for the tolerant genotypes (981 bp with OPA-03, 630 bp with OPA-07, 630 bp with OPA10, 1725 bp with OPJ01, 580 bp with OPM20 y 630 bp with OPJ05). OPA series allowed observation of higher levels of polymorphisms, having PIC values ranging from 0.22 y 0.68. Hierarchical conglomerate analysis separated genotypes in 2 main groups, one including genotypes with the A genome exclusively, and the other with the B genome, except for FHIA 17 in vitro. There were genetic differences between FHIA 17 growing under field conditions and the one regenerated in vitro.
Downloads
References
• Damasco, O. P., G. C. Grahan, R. J. Henry, S. W. Adkins, M. K. Smith and I. D. Godwin. 1996. Random amplified polymorphic DNA (RAPD) detection of dwarf off- types in micropropagated cavendish (Musa sp. AAA) bananas. Plant Cell Reports. 16:118-123.
• Dellaporta, S. J., J. Wood and J. B. Hicks. 1983. A plant DNA minipreparation: version II. Plant Mol. Rep. 1:19-21.
• García, E. y C. Giménez. 1999. El uso de marcadores moleculares en la detección de variantes somaclonales en Musa ssp. Mem. Inst. Biol. Exp. 2:115-118.
• Gubbuk, H., M. Pekmezci, A. N. Omus and M. Erkan. 2004. Identification and selection of superior banana phenotypes in the cultivar Dwarf Cavendish using characteristics and RAPD markers. Pakist. J Bot. 36:331-342.
• Howell, E. C., J. H. Newbury, R. Swennen, L. A. Wilhers and B. V. Ford-Lloyd. 1994. The use of RAPD for identifying and classifying Musa germplasm. Genome 37:328-332.
• Javed, M. A. and R. Y. Othman. 2005. Characterization of Fusarium wilt-resistant and Fusarium wilt- susceptible somaclones of banana cultivar Rastali (Musa AAB) by Random Amplified Polymorphic DNA and Retrotransposon markers. Plant Molec. Biol. Rep. 23:241-249.
• Javed, M. A., M. Chai and R. Y. Othman. 2004. Study of resistance to Fusarium oxysporum using RAPD markers. Biol. Plant. 48:93-99.
• Kaemmer, D., R. Afza, K. Weising, G. Kahl and F. J.Novak. 1992. Oligonucleotide and amplification fingerprinting of wild species and cultivars of banana (Musa spp.) Biotechnology 10:1 030-1 035.
• Lakshmanan, V., S. R. Venkataramareddy and B.Neelwarne. 2007. Molecular analysis of genetic stability in long-term micropropagated shorts of banana using RAPD and ISSR markers. E. J. Biotechnol. 10(1). Disponible en: https://bit.ly/39eJ1lf
• Martin, K. P., S. K. Pachathundikandi, C. L. Zhang, A.Slater and J. Madassery. 2006. RAPD analysis of a variant of banana (Musa sp.) cv. Grande naine and its propagation via shoot tip culture. In vitro Cell. Dev. Biol. Plant. 42:188-192.
• Nadal-Medina, R., G. Manzo-Sánchez, J. Orozco- Romero, M. Orozco-Santos y S. Guzmán-González. 2009. Diversidad genética de bananos y plátanos (Musa spp.) Determinada mediante marcadores RAPD. Revista Fitotecnia Mexicana 32(1):1-7.
• Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). 2004. FAOSTAT Agricultura. Superficie, producción y rendimiento. Disponible en: https://bit.ly/33IcH96
• Pérez Vicente, L. 2009. Enfermedades exóticas que constituyen una amenaza para la estabilidad de la producción de bananos y plátanos en América Latina y el Caribe. Fitosanidad 13(1):34-35.
• Pillay, N., D. C. Nwakanma and A. Tenkouano. 2000. Identification of RAPD markers to A and B genome sequences in Musa L. Genome 43:763-767.
• Pillay, M., E. Ougundidiwin, D. C. Nwakanma, G. Ude and A. Tenkouano. 2001. Analysis of genetic diversity and relationships in East African banana germoplasm. Theor Appl Genet. 102:965-970.
• Ramaje, C. M., A. M. Borda, S. D. Hamill and M. K. Smith. 2004. A simplified PCR test for early detection of dwarf off-types in micropropagated Cavendish bananas (Musa spp. AAA). Sci. Hort. 103:45-151.
• Ray, T., I. Dutta, P. Saha, S. Das and S. C. Roy. 2006. Genetic stability of three economically important micropropagated banana (Musa spp.) cultivars of lower Indo-Gangetic plains, as assessed by RAPD and ISSR markers. Plant Cell Tiss. Org. Cult. 85:11-21.
• Roldán-Ruiz, I., J. Dendauw, E. Van Bockstaele, A. Depicker and M. De Loose. 2000. AFLP Markers reveal high polymorphic rates in ryegrasses (Lollium spp.). Mol. Breed 6:125-134.
• Salazar, B., H. Laurentín, M. Dávila and M. Castillo. 2006. Reliability of the RAPD technique for germplasm analysis of sesame (Sesamum indicum L.) from Venezuela. Interciencia 31(6):456-460.
• Thu, N. X., L. T. L. Oanh y H. H. Nhi. 2002. Utilización de la técnica RAPD para la identificación y clasificación de algunos cultivares de banano en Vietnam. INFOMUSA 11(1):48-49.
• Tripathi, L., J. N. Tripathi y W. K. Tushemereirwe. 2004. Estrategias para la resistencia a la enfermedad de la marchitez bacteriana de los plátanos a través de la ingeniería genética. African Journal of Biotechnology 3(12):688-692.
• Tripathi, L. and J. N. Tripathi. 2009. Relative suscep- tibility of banana cultivars to Xanthomonas campes- tris pv. musacearum. African Journal of Biotechnology 8(20):5.343-5.350.
• Uma, S., S. A. Siva, M. S. Saraswathi, M. Manicka- vasagam, P. Durai, R. Selvarajan and S. Sathia- moorthy. 2006. Variation and intraspecific relationships in India wild Musabalbisiana (BB) population as evidenced by Random Amplified Polymorphic DNA. Gen. Resour. Crop Evol. 53:349-355.
• Vidal, M. C. and E. García. 2000. Analysis of Musa spp. somaclonal variant resistant to yellow sigatoca. Plant Molec. Biol. Rep. 18:23-31.
• Visser, A. H. 2001. Characterization of banana and plantain using random amplified polymorphic DNA markers. Horticult. Abstr. 72(2):1 154.
• Welsh, J. and M. McClelland. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 18:7 213-7 218.
• Williams, J., A. Kubelik, K. Livak, J. Rafalski and S. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nuc. Ac. Res. 18:6 531-6 535.
• Williams, J. G. K., M. K. Hanafey, J. A. Rafalski and S. V. Tingey. 1993. Genetic analysis using random amplified polymorphic DNA markers. Meth. Enzymol 218:704-740.
• Zambrano, A. Y., G. Martínez, Z. Gutiérrez, E.Manzanilla, J. L. Vicente-Villardón y J. Demey. 2007. Marcador RAPD asociado a la resistencia a Fusarium oxysporum en Musa. Interciencia 32(11):775-779.